House GOP Pushes FDA to Approve Smoke Free Alternatives
In a move aimed at providing adult smokers with alternative products, US Representative Richard Hudson and sixty of his House Republicans called upon the Biden administration to accelerate the approval process for smoke-free tobacco products through the Food and Drug Administration (FDA).
House Republicans Urge Expedited FDA Approval for Smoke-Free Tobacco Products
Led by Rep. Richard Hudson (R-NC), GOP representatives penned a letter to President Joe Biden, emphasizing the urgent need to approve new smoke-free nicotine products. This follows a letter by Senators Paul, Manchin, and Budd to the FDA urging reform and the approval of what they refer in their to as “harm-reduction products”.
The House contingent did not pull any punches when calling out failed FDA policies:
According to data from the Centers for Disease Control and Prevention, in the past only 1 in 10 adult smokers quit smoking. These consumers deserve to have alternative, less harmful products available to make informed choices of their own or may make it easier to quit. We cannot allow scare tactics and misinformation to keep 30 million adult smokers in the U.S. from making informed decisions and providing them with information about and access to alternative products that present less risk than continued smoking.
FDA Inactivity Endangers Americans
The letter argues it is the FDA's responsibility to facilitate access to nicotine-containing alternatives and that they have completely failed at this task.
"Rather than banning products that have proven effective in converting smokers or reducing cigarette consumption, the FDA must utilize the regulatory approval framework provided by Congress fifteen years ago. Since the Family Smoking Prevention and Tobacco Control Act of 2009 was signed into law by President Obama, the FDA has received over 26 million applications to market innovative smoke-free products. However, the FDA has authorized fewer than 50 product applications (of which less than 10 are commercially available products) and many other applications have been waiting over three years for official review. Meanwhile, during this same period, FDA has authorized thousands of combustible cigarette product applications, as measured by CTP’s own performance metrics."
The current backlog of more than 26 million applications for smoke-free products, with a meager 50 approvals to date, signals a pressing need for expediency in the FDA's review process. There is certainly a fact pattern present with the PMTA process that closely resembles regulatory capture.
Regulatory capture is a form of corruption where policymakers or regulator are co-opted to serve the commercial, ideological, or political interests of a minor constituency, such as a specific industry or special interest group.

Additionally, the letter draws attention to the trend of illicit nicotine-containing products entering the U.S. from markets like China. The fact even Big Tobacco owned Vuse and NJOY relies on Chinese components and merely “blend” their e-liquid ingredients in the US lays bare the damage these regulations have done to the US worker.
Millions of Vape Applications Languish as Cigarettes Approved
Despite the substantial volume of smoke-free product applications, the FDA has been criticized for prioritizing combustible product approvals over alternatives. The letter from the GOP contingent weighed in again:
This is a matter of public health. According to an ever-increasing amount of scientific research, smoke-free products hold enormous potential for current smokers to quit by switching to these less harmful products, and we urge your administration to encourage the FDA to review smoke-free product applications more effectively and efficiently and to make those determinations on sound science.
Acknowledging the imperative to safeguard against underage use, Republican lawmakers promoted robust regulatory measures while simultaneously advocating for expanded access to proven smoke-free alternatives. Especially as flavor bans have been shown to increase cigarette sales.
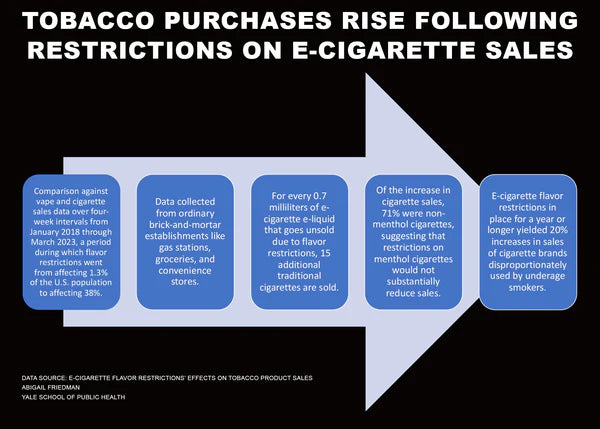
Emphasizing the role of sound scientific evaluation in regulatory decision-making, the letter urges the Biden administration to prioritize efficiency and effectiveness in the FDA's review process.
Schumer Wants to Deny Adults Smoke Free Alternatives
The call for expedited FDA approval comes amidst broader discussions surrounding nicotine regulation, with Senate Majority Leader Chuck Schumer's recent push for stricter oversight of synthetic nicotine products like Zyn sparking debate. The letter addresses Schumer’s most recent quest.
In recent weeks there have been calls on the FDA to ban certain nicotine-containing products due to the potential of underage use. There is no debate: youth should not have access to nicotine-containing products, nor should youth be subject to marketing or advertising of these products on social media. Congress has taken several steps to help address these challenges, including raising the legal age to purchase tobacco and nicotine containing products to 21 years of age and bringing synthetic nicotine products under the purview of FDA ands its mission to protect and promote public health.
FDA Refuses to Follow the Science
Rep. Hudson stresses the importance of providing Americans with viable alternatives to traditional smoking on his Congressional website, condemning bureaucratic delays in the FDA's approval process.
Criticism of the Biden administration's approach to tobacco regulation extends beyond smoke-free products, with concerns raised about the potential ramifications of a proposed menthol cigarette ban. Lawmakers and advocacy groups caution against unintended consequences of their absolutist and prohibitionist approach to an issue with many layers and nuance.
US vape companies filed an Amicus Brief to SCOTUS. It regarded the Supreme Court recently hearing the case Loper Bright Enterprises v. Raimondo. Their decision that has the potential to roll back the massive and unchallenged powers of federal regulators, allowing private businesses recourse when battling unelected federal regulators with a blank to check to interpret statutes.
What cannot be ignored is that the FDA’s PMTA scheme has destroyed countless jobs, and approved only a handful of obsolete e-cigarettes, all manufactured by the tobacco industry.

Whether this is due to regulatory capture or outsized pressure by Senators, it is growing increasingly difficult to claim they are following the science.
Big Tobacco Pushes for PMTA Registry Laws
Regulations that bar alternative products, leave tobacco industry products with a virtual monopoly. This is why it is tobacco giant Altria that is pushing tirelessly for PMTA Registry laws, an issue we discuss at length in the feature Big Lies by Big Tobacco.
Their motivations for supporting a PMTA scheme that leaves cigarettes and a handful of obsolete vapes on their manufacture on store shelves was outlined in a shareholders discussion published by Barclays.

It is notable that vaping consumers pay a very heavy price outside of being denied access to the flavors they prefer. A Vuse pod kit and the typical disposable vape both use Chinese hardware. Vuse manufacturing was moved out the US almost a decade ago. These even share suppliers and manufacturing facilities.
RJR's Vuse can claim to "blend" e-liquids in the US but they certainly don't say these are US-made e-liquids sourced from US ingredients. Yet despite this shared DNA, there is a huge discrepancy at the cash register.
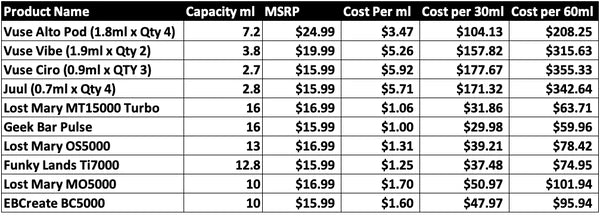
Vape Bans Punish Marginalized Groups
Big Tobacco's vested interest in snuffing out the vaping industry raises questions as to who benefits from flavor bans and why adults from marginalized communities must suffer when youth vaping rates have dropped 60 percent since tobacco-21 and Trump's ban on prefilled pods with flavors were implemented.
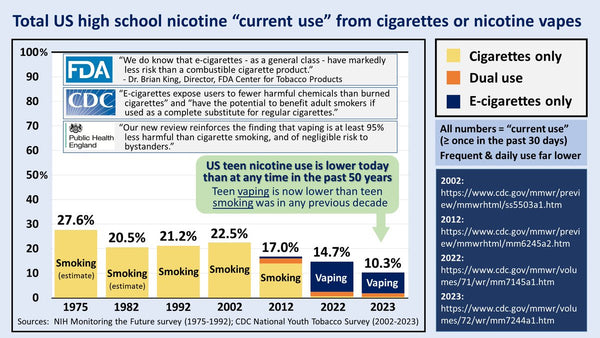
Regardless of age, the vast majority of vapers prefer what the FDA calls characterizing flavors. These of course are the very flavors Democratic Senators have been hell-bent on banning for years.
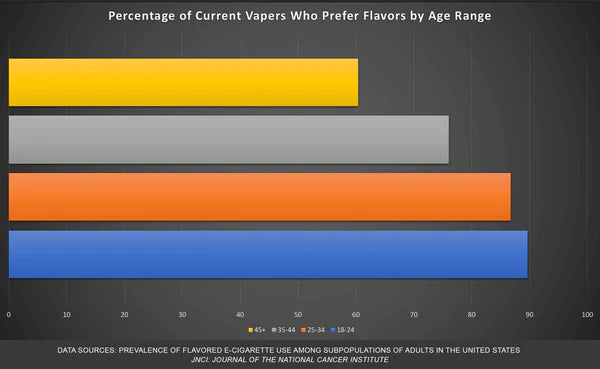
These are also common artificial fruit flavors found in everything from soda pop (another Bloomberg target) and hard seltzers to kombucha. The number of popular consumable products flavored with artificial tobacco notes is significantly shorter. As the infographic below shows, this plays out in real terms when adults look at making the switch.
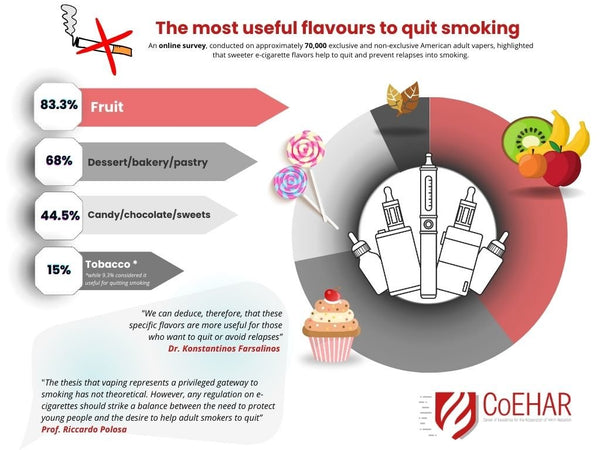
Politically, this decision may make sense as Bloomberg has spent around a quarter billion dollars to ban vaping.
Ethically it is questionable. Recently the Center for Black Equity called on the FDA to approve a wide range of e-cigarettes to ensure Black and LGBTQIA+ Americans can access effective options.
Will FDA Regulations Face Additional Court Challenges?
Courts generally have refrained from questioning the regulatory processes of the FDA, due to the Chevron Doctrine. The Chevron defense compels federal courts to defer to the judgment of the experts at regulatory agencies. Especially in cases where the statute is unclear.
This is a major reason why vape companies that have challenged the FDA’s regulatory overreach have achieved only mixed results. The need for swift action action against these failed FDA policies underscores the urgency of addressing regulatory barriers and a 5th Circuit Court victory for the vape industry was a rare beacon of hope.
Their summary of the PMTA process provides describes a textbook case of bureaucratic failure.







